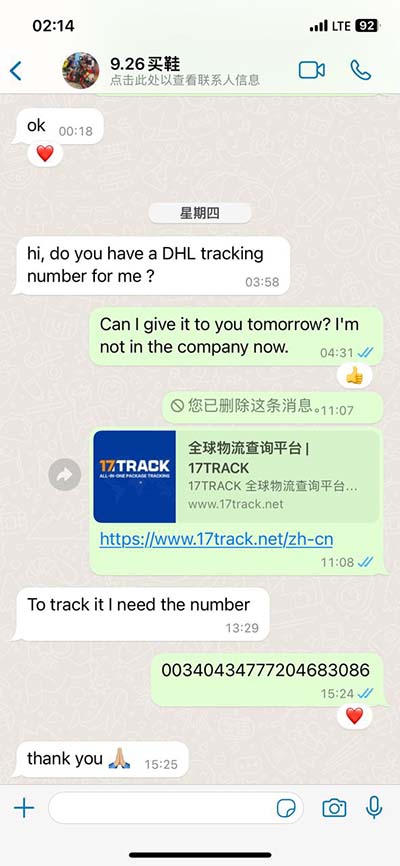
lv formulation and filling | formulation development strategies lv formulation and filling the filling volume of the respective therapeutics can vary. At IDT Biologika filling of different volumes and primary packaging systems is possible for example, the filling of 0.5 – 3 mL syringes and 2R up to 25R vials can be per-formed. Chemical interactions like binding of (viral) pro-teins on surfaces of primary packaging material can be a Alfa Romeo: T: 22692215: E:
[email protected]: Fiat: T: 22692213/4: E:
[email protected]: Hyundai: T: 22692201/4: E:
[email protected]: Jeep: T: 22692215: E:
[email protected]: Kia: T: 22692120/3: E:
[email protected]: Opel: T: 22692122: E:
[email protected]: .
0 · rational formulation for aav
1 · formulation for aav vectors
2 · formulation development strategies
3 · formulation development in manufacturing
4 · aav formulation in manufacturing
5 · aav formulation development
Une balade urbaine pour découvrir le patrimoine d’Albi. Caractéristiques. Distance : 5km. Durée : 2h. Dénivelé : 50m. Difficulté : Facile. En empruntant les ruelles chargées d’histoire de la cité épiscopale d’Albi, classée au patrimoine mondial par l’UNESCO, découvrez les mille et une richesses du chef-lieu du Tarn .
the filling volume of the respective therapeutics can vary. At IDT Biologika filling of different volumes and primary packaging systems is possible for example, the filling of 0.5 – 3 mL . Filling facilities commonly used for manufacturing small volume gene therapy products are semi-automated, raising concerns regarding errors associated with crimping and .the filling volume of the respective therapeutics can vary. At IDT Biologika filling of different volumes and primary packaging systems is possible for example, the filling of 0.5 – 3 mL syringes and 2R up to 25R vials can be per-formed. Chemical interactions like binding of (viral) pro-teins on surfaces of primary packaging material can be a Filling facilities commonly used for manufacturing small volume gene therapy products are semi-automated, raising concerns regarding errors associated with crimping and sealing. Low-shear filling operations such as peristaltic pump technology in closed systems would be advantageous.
Chimeric antigen receptor (CAR) T-cell products are commonly generated using lentiviral vector (LV) transduction. Optimal final formulation buffer (FFB) supporting LV stability during cryostorage is crucial for cost-effective manufacturing.
Formulation, final filtration, and filling are the last steps in viral vector production. A rational formulation design developed by optimizing solution conditions and high-quality excipients can significantly increase viral vector stability and shelf-life. Cassettes and HFs, with different cut-offs, were evaluated for virus concentration and formulation. No correlation was observed in the recovery yield with the MWCO and TMP variations, and different devices were proven suitable for LV concentration and formulation.Preparing stable viral vectors, preventing their degradation during manufacturing, handling, and storage, and maintaining their long-term stability and ef cacy are major challenges for the AAV manufacturer.
Dissimilar to other retroviral vectors, in particular those derived from gammaretroviruses (formerly known as oncoretroviruses), lentiviral vectors (LV) can mediate gene transfer into non-dividing cells, e.g. stem cells, lymphocytes, dendritic and nerve cells.
“Multiple steps are involved in upstream (e.g., seed train, infection/transfection, harvest), downstream (e.g., cell lysis, chromatography, filtration, and formulation), and fill/finish (e.g., filling and freeze drying) processes, with each step bearing the risk of losing sterility,” says Karl Heller, vice-president CMC development with . Enhance your formulation for a stable viral vector product – compare and contrast viral vector process development for cell and gene therapies; Reduce impurity and maximize process development robustness for greater process control and quality; Navigate the fast-evolving regulatory landscape to achieve GMP during scale-up and scale-outSterile fill-finish services. Efficiently complete the manufacturing process for your parenteral, injectable medicines. As a proven leader in the production of biologics and small molecules from first-in-human (FIH) trials to commercial-scale manufacturing, we can .the filling volume of the respective therapeutics can vary. At IDT Biologika filling of different volumes and primary packaging systems is possible for example, the filling of 0.5 – 3 mL syringes and 2R up to 25R vials can be per-formed. Chemical interactions like binding of (viral) pro-teins on surfaces of primary packaging material can be a
Filling facilities commonly used for manufacturing small volume gene therapy products are semi-automated, raising concerns regarding errors associated with crimping and sealing. Low-shear filling operations such as peristaltic pump technology in closed systems would be advantageous. Chimeric antigen receptor (CAR) T-cell products are commonly generated using lentiviral vector (LV) transduction. Optimal final formulation buffer (FFB) supporting LV stability during cryostorage is crucial for cost-effective manufacturing.Formulation, final filtration, and filling are the last steps in viral vector production. A rational formulation design developed by optimizing solution conditions and high-quality excipients can significantly increase viral vector stability and shelf-life. Cassettes and HFs, with different cut-offs, were evaluated for virus concentration and formulation. No correlation was observed in the recovery yield with the MWCO and TMP variations, and different devices were proven suitable for LV concentration and formulation.
Preparing stable viral vectors, preventing their degradation during manufacturing, handling, and storage, and maintaining their long-term stability and ef cacy are major challenges for the AAV manufacturer.
rational formulation for aav
formulation for aav vectors


Dissimilar to other retroviral vectors, in particular those derived from gammaretroviruses (formerly known as oncoretroviruses), lentiviral vectors (LV) can mediate gene transfer into non-dividing cells, e.g. stem cells, lymphocytes, dendritic and nerve cells.
“Multiple steps are involved in upstream (e.g., seed train, infection/transfection, harvest), downstream (e.g., cell lysis, chromatography, filtration, and formulation), and fill/finish (e.g., filling and freeze drying) processes, with each step bearing the risk of losing sterility,” says Karl Heller, vice-president CMC development with .
Enhance your formulation for a stable viral vector product – compare and contrast viral vector process development for cell and gene therapies; Reduce impurity and maximize process development robustness for greater process control and quality; Navigate the fast-evolving regulatory landscape to achieve GMP during scale-up and scale-out

formulation development strategies
formulation development in manufacturing
38 items. Sort: Featured. Alexander McQueen. Oversized Sneaker (Men) $354.00. (40% off) $590.00. ( 5) Only a few left. Alexander McQueen. Oversized Sneaker (Men) $590.00. ( 58) New! Alexander McQueen. .
lv formulation and filling|formulation development strategies